
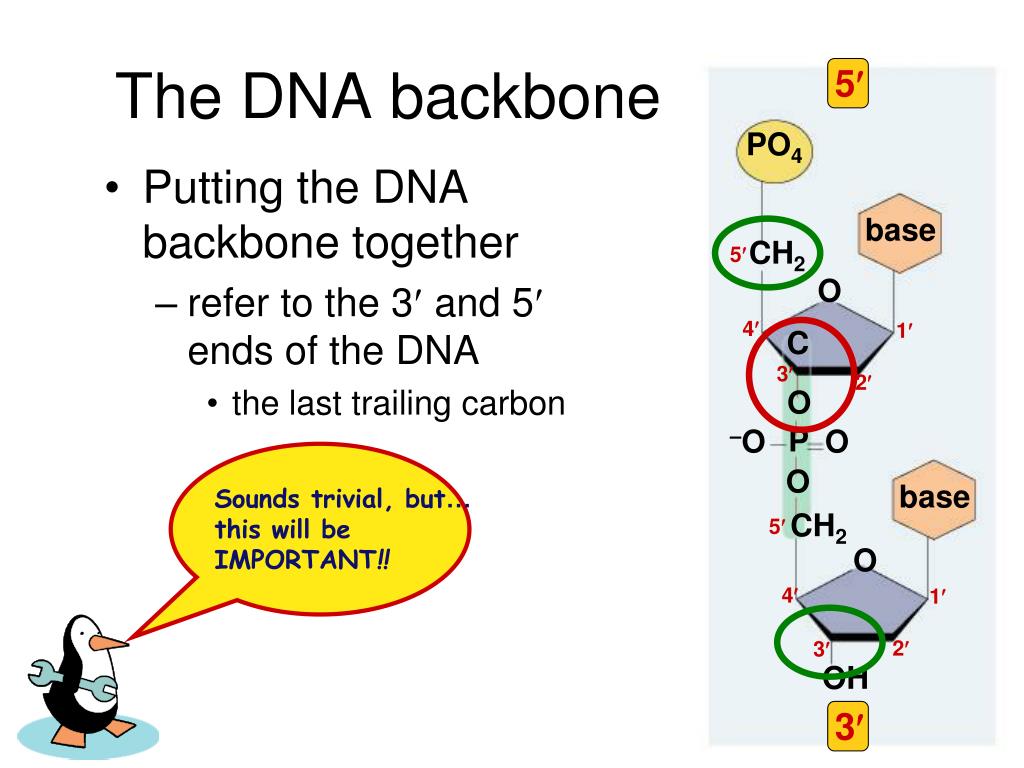
The first atom visible is atom 5, a carbon attached to the deoxyribose ring. Atom 10 is a carbon (carbon 1'), which is where the actual DNA base is attached.įor the first DNA segment in a chain, the phosphate group is missing. Atom 9 is an oxygen which joins the phosphate group of the next DNA segment.
Foldit considers one of the oxygen atoms of the phosphate to belong to the deoxyribose of the preceding segment, so only the oxygen atoms 2 through 4 are numbered.Ītom numbering continues with the dexoyribose group. Foldit starts numbering with the phosphorous in the phosphate group. For example, the C terminal of a chain has an extra backbone atom, so it's beta carbon, the first atom of the sidechain, is 6 instead of 5.įor DNA, the backbone atoms always have the same number, regardless position in the chain. In a protein, the number of atoms in a segment is different depending on its position in the chain. The rules for numbering atoms are different in DNA, however. Just as in a protein, Foldit can identify atom numbers in DNA. The statements below are based on the similarities between RNA and DNA. DNA has only been found in intro puzzles to date, which don't allow the use of the recipes used to verify RNA atom numbering. The hydroxyl group is what distinguishes RNA from DNA.Ĭaution: atom numbering for DNA has not been validated. In RNA, a hydroxyl group (oxygen and hydrogen) is attached to carbon 2'. The DNA base is attached to carbon 1' of the deoxyribose. The carbon atoms are referred to as 1', 2', 3' and 4'. The sugar group in the DNA backbone is always a form of deoxyribose, a pentagonal ring consisting of an oxygen atom and four carbon atoms. Each segment of DNA or RNA is linked to the next by a phosphate. A phosphate group consists of a phosphorous atom bound to four oxygen atoms. This is why "RNA" stands for "ribonucleic acid", and "DNA" stands for "deoxyribonucleic acid".īoth DNA and RNA backbone consists of alternating groups phosphates and sugars. The difference is that RNA backbone contains the sugar ribose, while DNA backbone contains the sugar deoxyribose. The blue numbers 1' to 4' refer to the carbons in the in deoxyribose ring.ĭNA backbone is more complicated than the backbone of the amino acids in a protein.ĭNA backbone is highly similar to RNA backbone. DNA ligase is able to form a phosphodiester bond between the nucleotides.DNA backbone showing chemical groups. The relative ease of RNA hydrolysis is an effect of the presence of the 2' hydroxyl group.Ī phosphodiesterase is an enzyme that catalyzes the hydrolysis of phosphodiester bonds, for instance a bond in a molecule of cyclic AMP or cyclic GMP.Īn enzyme that plays an important role in the repair of oxidative DNA damage is the 3'-phosphodiesterase.ĭuring the replication of DNA, there is a hole between the phosphates in the backbone left by DNA polymerase I. The phosphodiester linkage between two ribonucleotides can be broken by alkaline hydrolysis, whereas the linkage between two deoxyribonucleotides is more stable under these conditions. Hydrolysis of phosphodiester bonds is catalyzed by phosphodiesterases, which are involved in repairing DNA sequences. In order for the phosphodiester bond to be formed and the nucleotides to be joined, the tri-phosphate or di-phosphate forms of the nucleotide building blocks are broken apart to give off energy required to drive the enzyme-catalyzed reaction. The negative charge attracts histones, metal cations such as magnesium, and polyamines. Repulsion between these negative charges influences the conformation of the polynucleic acids. Phosphodiesters are negatively charged at pH 7. These saccharide groups are derived from deoxyribose in DNA and ribose in RNA.

Specifically, the phosphodiester bond links the 3' carbon atom of one sugar molecule and the 5' carbon atom of another (hence the name, 3', 5' phosphodiester linkage ). The 3' carbon of one sugar is bonded to the 5' phosphate of the adjacent sugar. The phosphate is attached to the 5' carbon. Phosphodiester bonds make up the backbones of DNA and RNA. Discussion of phosphodiesters is dominated by their prevalence in DNA and RNA, but phosphodiesters occur in other biomolecules, e.g. The "bond" involves this linkage C−O−PO − 2O−C. In chemistry, a phosphodiester bond occurs when exactly two of the hydroxyl groups ( −OH) in phosphoric acid react with hydroxyl groups on other molecules to form two ester bonds. The 5' end has a 5' carbon attached to a phosphate, and the other end, the 3' end, has a 3' carbon attached to a hydroxyl group. Diagram of phosphodiester bonds ( PO 3− 4) between three nucleotides.


 0 kommentar(er)
0 kommentar(er)
